Hetero announces approval and launch of Movfor (Molnupiravir 200 mg)
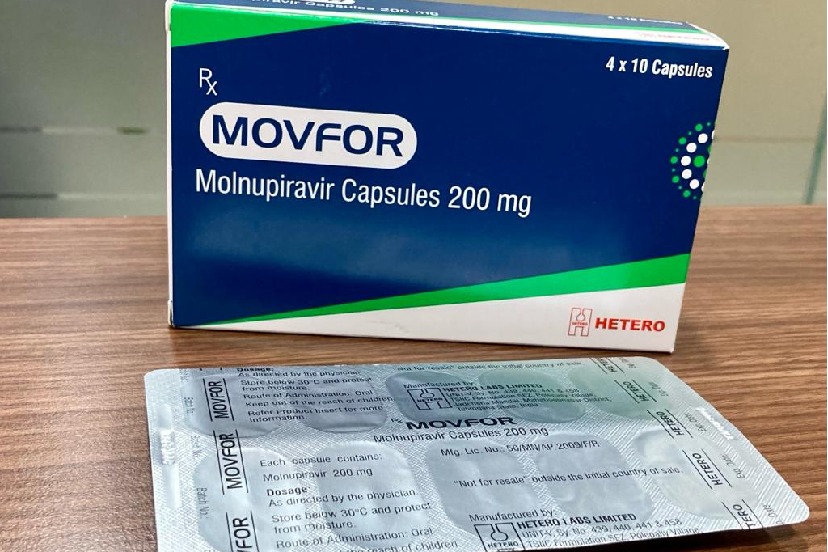
- Hetero announces approval and launch of Movfor (Molnupiravir 200 mg) to treat high-risk adult patients with COVID-19
Hetero’s Movfor will be made available in a 40 capsule pack (200 mg per capsule) and will be marketed by its associate company ‘Hetero Healthcare’ in India with the support of its strong distribution network across the country.
Dr. B. Partha Saradhi Reddy, Chairman, Hetero Group of Companies, said: “This approval consolidates India’s efforts to address the world’s greatest health threats, i.e., COVID-19. Improving access to critical medicines will always remain the highest of priorities to us."
Under the aegis of ‘Make in India’ campaign, commercial production of ‘Movfor’ will be undertaken at Hetero’s world-class facilities in Telangana and Himachal Pradesh, where it is ensured to meet highest quality standards.